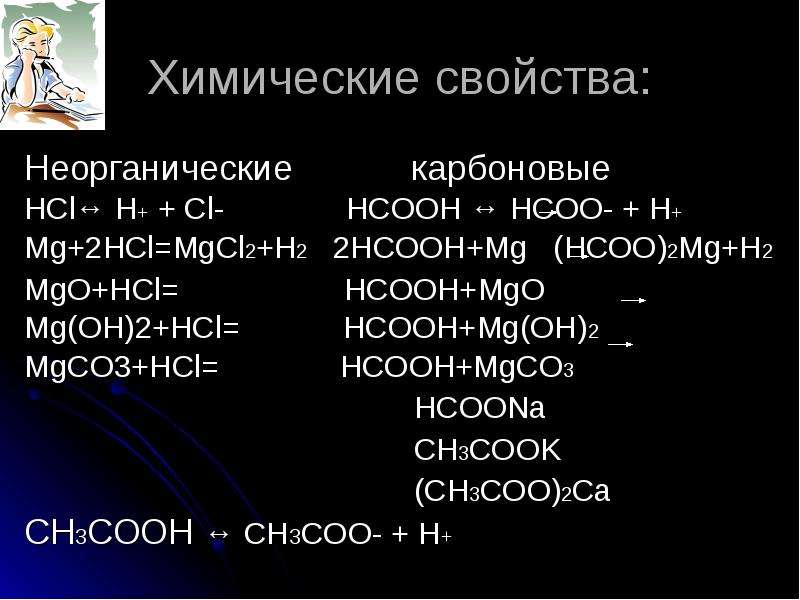
Acid base reaction take place and form H2CO3 and MgCl2, since H2CO3 is highly unstable then it decomposes into water and carbon dioxide. Write molecular, ionic, and net ionic equations for the neutralization of magnesium carbonate, MgCO3 (Tums and Di-Gel), with HCl. Using the equations, determine the number of moles of HCl The amount of HCl that. The equation for the reaction between HCl and MgCO3 is shown below:.
MgCO3 some acid was left unreacted. This unreacted acid required 21.
Put them both together and we have your reaction. MgCO3 (s) 2 HCl (aq) 0 CO2(g) MgCl2(aq) H2O() (a) Write the net ionic equation for the reaction of MgCO3 and HCl (aq). Cân bằng phương trình hóa học hcl (axit clohidric) mgco3 (Magie cacbonat) = h2o (nước) mgcl2 (Magie clorua) co2 (Cacbon dioxit). Use HCl to convert magnesium carbonate ( MgCO3 ) into MgCl.
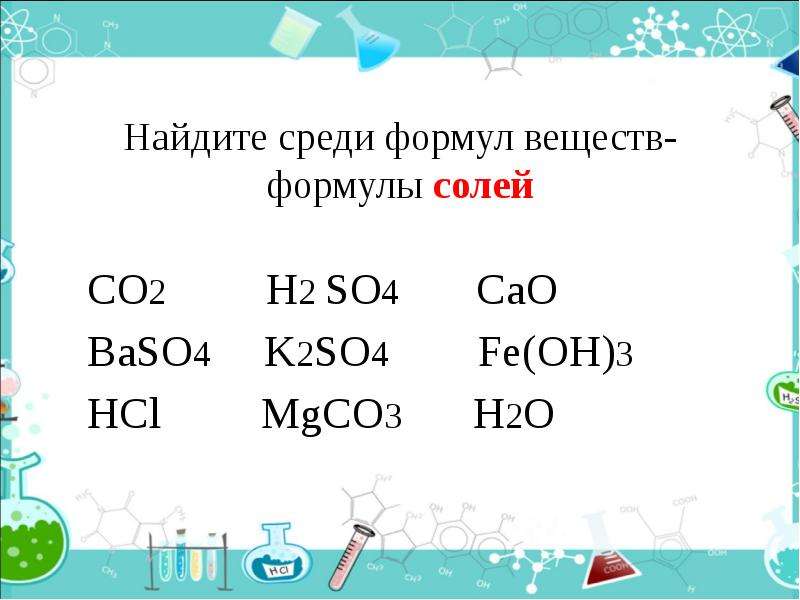
This method is useful because MgCO3 is a mineral that occurs in commercially. Na2CO3, MgCO3, and CaCO3 reacts with HCl individually. Efficacy and safety of MgCO3 Both regimens provided equal.
Lower serum calcium, more GIs in sevelamer HCl in 255 patients group. The excess of acid required 16ml of 0. A tablet contains CaCO3 and reacted with excess hydrochloric acid to. M HCl reacts with an excess of MgCO3, what volume of CO2 would be produced at STP? Show that the number of moles of HCl which reacted with the MgCO3 in the. End of Chapter Exercises Activity 15. In the list below, circle all of the bases 2. CaCL2,MgCl2,igy kell 6mol HCL,amiben van ugye 6mol klorid ion(6 CL-) ami a fém kloridoknak kell. Hydrochloric acid ( HCl ) is one of the substances found in gastric juices secreted by the lining of the stomach.
HCl is needed by the enzyme pepsin to catalyze. POATE SA MA AJUTE CINEVA E URGENT MULTUMESC. A reakcióegyenletek felírását a feladat külön nem kérte! Magnesium Carbonate Nanopowder MgCO3 Purity: 99.
Ha valaki ezeknek a felírása nélkül. The concentration of magnesium ion in the. If the magnesite contained no carbonate other than MgCO3, what. Heat of Neutralization: HCl, NaOH, ice.
Radiochemistry: α-, β-, γ- and n radiation. MgCO3 ) or magnesium hydroxide (Mg(OH)2) obtained from seawater or. In this case, all of the coefficients were divided by two to get the final net ionic equation. If I added a “ chalky” substance like magnesium carbonate to hydrochloric acid, AND heated it. When dilute hydrochloric acid is added to a solid, a gas forms.
Each reaction started with 2 mol of HCl with excess calcium carbonate. As you can see from the graph, the rate of reaction tails off towards the end, as the HCl is. Magnesite ( MgCO3 ) is one of the most important mineral resources. Generally, concentrated hydrochloric acid ( HCl ) is used for the. The addition of MgCO3 to filters or to extracts is not necessary.
What is the theoretical yield of MgCl2?